`=>` Nyholm and Gillespie (1957) refined the VSEPR model by explaining the important difference between the lone pairs and bonding pairs of electrons.
● While the lone pairs are localised on the central atom, each bonded pair is shared between two atoms.
● As a result, the lone pair of electrons in a molecule occupy more space as compared to the bonding pairs of electrons.
● This results in greater repulsion between lone pairs of electrons as compared to the lone pair - bond pair and bond pair - bond pair repulsions.
● These repulsion effects result in deviations from idealised shapes and alterations in bond angles in molecules.
`=>` For the prediction of geometrical shapes of molecules with the help of VSEPR theory, it is convenient to divide molecules into two categories as :
(i) molecules in which the central atom has no lone pair and (ii) molecules in which the central atom has one or more lone pairs.
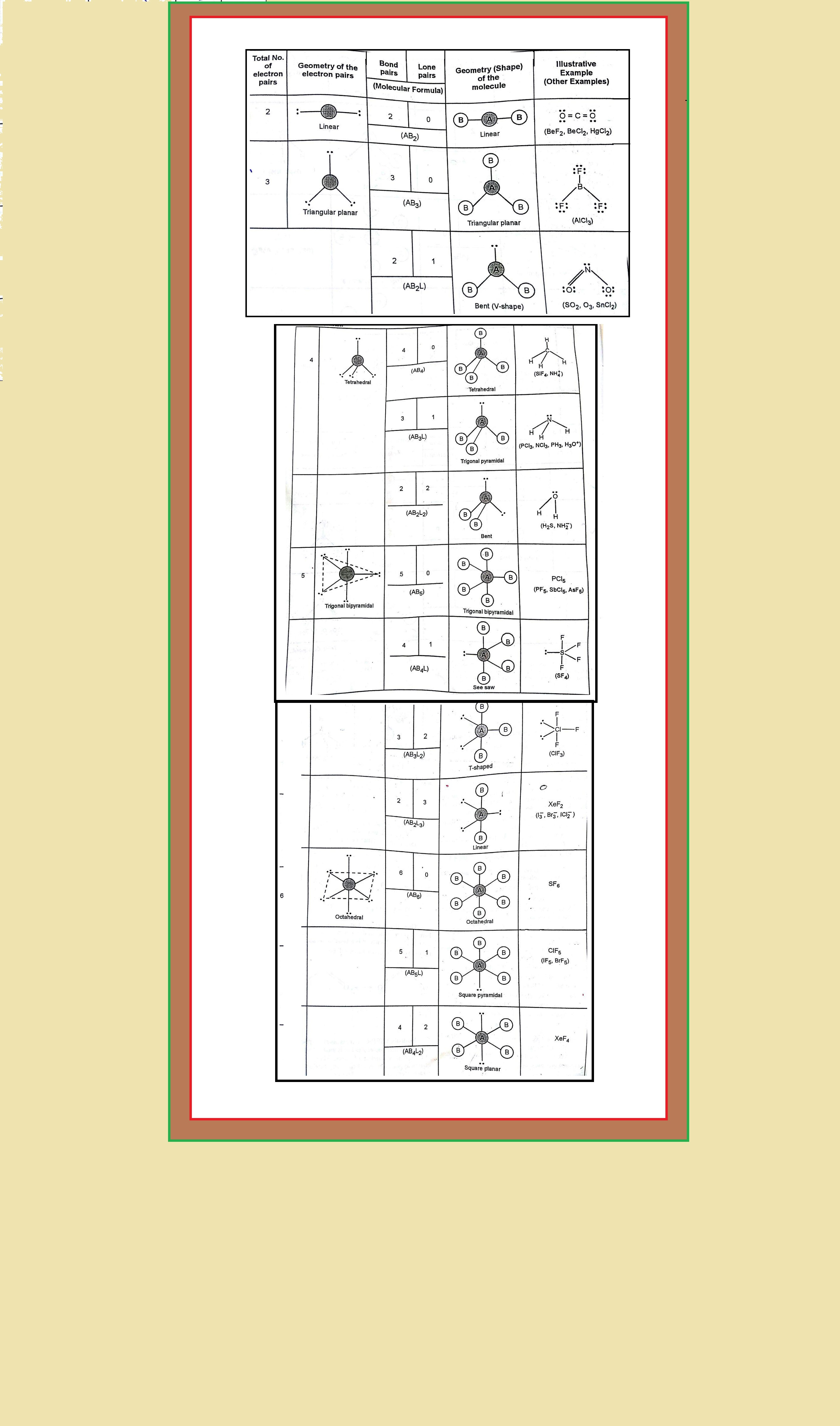
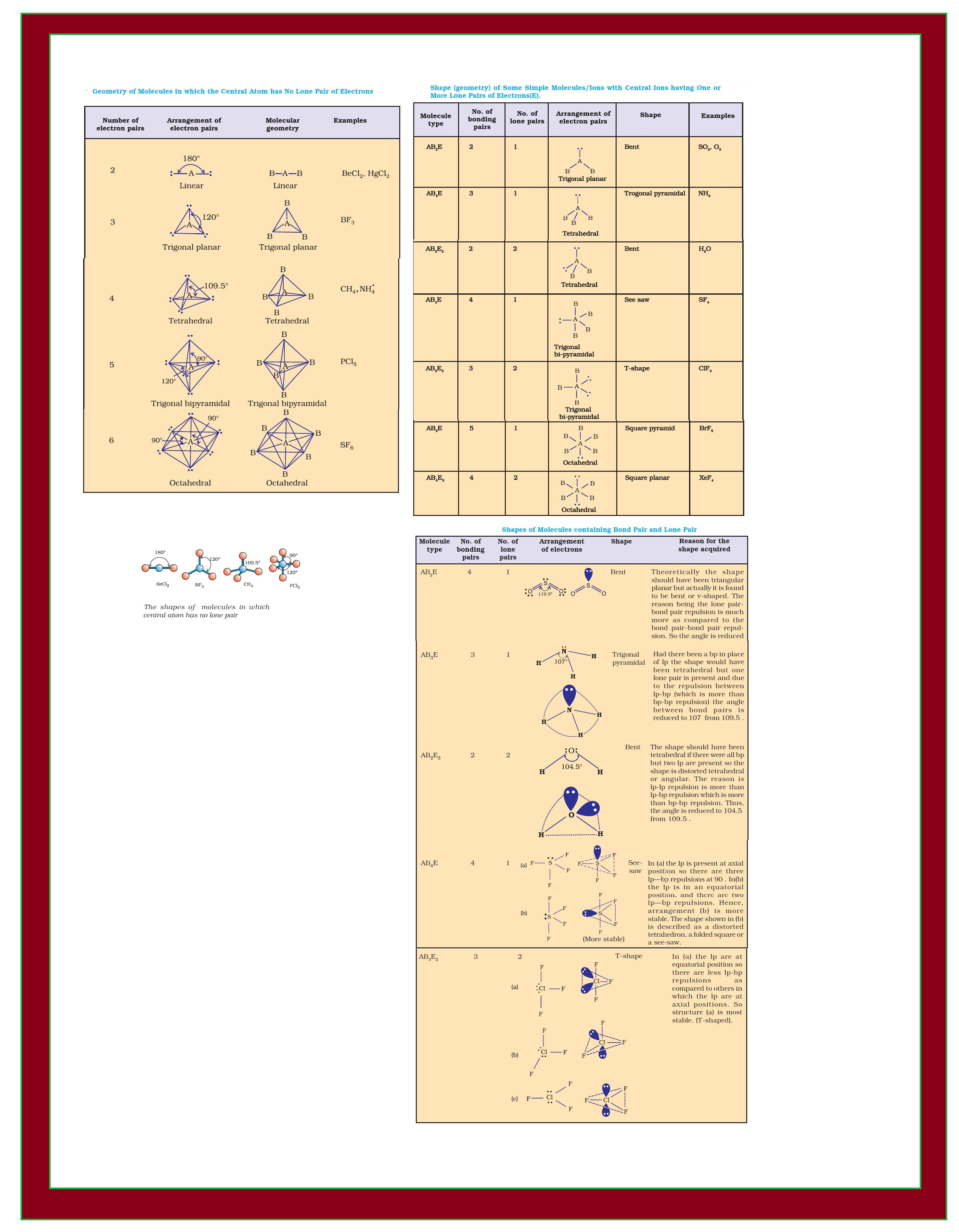
`=>` Table shows the arrangement of electron pairs about a central atom `A` (without any lone pairs) and geometries of some molecules/ions of the type `AB`.
`=>` Table shows shapes of some simple molecules and ions in which the central atom has one or more lone pairs.
`=>` Table explains the reasons for the distortions in the geometry of the molecule.
`=>` As depicted in Table in the compounds of `AB_2`, `AB_3`, `AB_4`, `AB_5` and `AB_6`, the arrangement of electron pairs and the `B` atoms around the central atom `A` are : linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral, respectively.
● Such arrangement can be seen in the molecules like `BF_3 (AB_3), CH_4 (AB_4)` and `PCl_5 (AB_5)` as depicted below by their ball and stick models.
`=>` The VSEPR Theory is able to predict geometry of a large number of molecules, especially the compounds of `p`-block elements accurately.
`=>` It is also quite successful in determining the geometry quite-accurately even when the energy difference between possible structures is very small.
`color{green}("Disadvantage :")` The theoretical basis of the VSEPR theory regarding the effects of electron pair repulsions on molecular shapes is not clear.
`=>` Nyholm and Gillespie (1957) refined the VSEPR model by explaining the important difference between the lone pairs and bonding pairs of electrons.
● While the lone pairs are localised on the central atom, each bonded pair is shared between two atoms.
● As a result, the lone pair of electrons in a molecule occupy more space as compared to the bonding pairs of electrons.
● This results in greater repulsion between lone pairs of electrons as compared to the lone pair - bond pair and bond pair - bond pair repulsions.
● These repulsion effects result in deviations from idealised shapes and alterations in bond angles in molecules.
`=>` For the prediction of geometrical shapes of molecules with the help of VSEPR theory, it is convenient to divide molecules into two categories as :
(i) molecules in which the central atom has no lone pair and (ii) molecules in which the central atom has one or more lone pairs.
`=>` Table shows the arrangement of electron pairs about a central atom `A` (without any lone pairs) and geometries of some molecules/ions of the type `AB`.
`=>` Table shows shapes of some simple molecules and ions in which the central atom has one or more lone pairs.
`=>` Table explains the reasons for the distortions in the geometry of the molecule.
`=>` As depicted in Table in the compounds of `AB_2`, `AB_3`, `AB_4`, `AB_5` and `AB_6`, the arrangement of electron pairs and the `B` atoms around the central atom `A` are : linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral, respectively.
● Such arrangement can be seen in the molecules like `BF_3 (AB_3), CH_4 (AB_4)` and `PCl_5 (AB_5)` as depicted below by their ball and stick models.
`=>` The VSEPR Theory is able to predict geometry of a large number of molecules, especially the compounds of `p`-block elements accurately.
`=>` It is also quite successful in determining the geometry quite-accurately even when the energy difference between possible structures is very small.
`color{green}("Disadvantage :")` The theoretical basis of the VSEPR theory regarding the effects of electron pair repulsions on molecular shapes is not clear.